 CYDELIC
CYDELIC
 CYDELIC
CYDELIC

We provide sleek biomedical grade wearable devices optimized for precision data gathering.
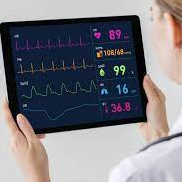
All of our devices provide real-time data streaming to our session monitoring console.

you get detailed reports from every session to track longitudinal changes in behavior.


Cydelic forms as an LLC and begins testing wearable hardware candidates for our session monitoring prototype. Significant biomarkers for exploration include EEG, heart activity, respiration, skin temperature, and emotional arousal.

Cydelic enters into partnership agreements with multiple vendors in the commercial biosensor space. Partnership agreements include access to device SDKs to allow Cydelic access to real-time monitoring options. Cydelic begins testing and benchmarking data output specifications for each device.

Cydelic delivers a functional prototype of the Session Monitoring Platform compatible with multiple hardware partners. Cydelic performs a demonstration of the session monitoring prototype for potential clinical partners, including real-time streaming of biodata for remote monitoring.

Cydelic forms a Class C Corporation in Delaware and begins formation of advisory board. Cydelic files our first provisional patent for our proprietary Dose Response Predictor, and attends the Wonderland conference in Miami to meet with potential investors and clinical partners.
Cydelic signs letter of intent to support Emyria in upcoming phase 2B clinical trial of MDMA-assisted psychotherapy for PTSD. This study will be the first-ever psychedelic clinical trial to gather biodata from wearable devices throughout the entire course of months-long therapy.

CEO / Senior Developer
Author of Psychedelic Information Theory

CTO / Hardware Engineer
Co-Founder of OpenBCI

Neuroscience Advisor
Inventor of the EmotiBit

EEG Advisor
Founder Brainstar Innovations

R&D Advisor
Pharmaceutical Senior Executive